ACADEMIA
Materials Modeling Shows Big Future for Boron Nitride Nanoribbons
Oak Ridge National Laboratory’s (ORNL’s) Alejandro Lopez-Bezanilla was smiling as Konstantin Novoselov, the 2010 Nobel Prize co-winner in physics, lectured at this year’s American Physics Society meeting.
Novoselov’s topic was graphene, the current darling of the nanoelectronic world. The reason for Lopez-Bezanilla’s smile? He was already months deep into a computational study of the material being proposed as a graphene substrate: the compound boron nitride.
Graphene, which is carbon in the form of freestanding 1-atom-thick sheets, is a natural for next-generation computer chips, communications equipment, and solar energy devices. Electrons flow through the material at an astonishing 1 million meters per second.
To live up to its potential, however, graphene needs support. On its own, its edges wrinkle, tear, or roll up. The silicon dioxide substrate used for today’s microchips is not a good partner for graphene; it creates vibrations that slow the electrons, and its surface is too bumpy.
An ideal substrate would not physically interfere with the graphene and would have a low electrical resistance. “The substrate has to be a dielectric material, a material which is insulating and that can be polarized [positive and negative charges grouped in two distinct regions] by an applied electrical field,” says Lopez-Bezanilla, a research associate in ORNL’s Computing and Computational Sciences Directorate. He is funded by the Petascale Initiatives program of the Department of Energys Office of Advanced Scientific Computing Research.
Any substrate will affect the electrons in the adjacent graphene layer, but boron nitride interferes less than silicon dioxide. Also, boron nitride resists chemical change and is unaffected by high temperatures, leading many researchers to believe that it could be that consummate base.
“Boron nitride is a covalent material with atoms tightly bonded to each other, but it also presents a strong ionic behavior,” explains Lopez-Bezanilla, making it a great insulator and poor conductor. With the help of computer simulations run on Jaguar, ORNL’s petascale supercomputer, Lopez-Bezanilla took a closer look—a nanoscale look—at boron nitride’s properties.
Boron nitride, like graphene, can be formed as one-atom thick sheets or as nanotubes, then cut into nanoribbons with their atoms arranged in a hexagonal lattice (imagine chicken wire).
After a boron nitride nanotube is cut, the resulting edge shape will affect the behavior of the nanoribbon. A slice straight through the lattice halves every other hexagon, making armchair-shaped edges (aBNNR); zigzagged (zBNNR) edges are made by cuts along the hexagonal borders. The zigzag-cut ribbon has one edge lined with nitrogen atoms and the other with boron atoms. A boron nitride nanoribbon (BNNR) is a quasi-one-dimensional structure, one atom thick, with a width-to-length ratio of 1 to 1,000-plus.
The size of a nanoribbon can be compared with a strand of spider’s silk, which is one thousand times thicker than one BNNR. If the BNNR were scaled up to the thickness of the spider silk filament, a correspondingly scaled silk strand would be as wide as a coin’s edge.
Changing boron nitride’s properties
The judicious application of elements to a material—a process known as functionalization—can change the material properties at the nanoscale. Lopez-Bezanilla pushed boron nitride’s boundaries by modeling the compound under different conditions to see what new properties it might have at the nanoscale, expanding its usefulness. Working with colleagues at ORNL, he simulated zBNNRs modified by oxygen or sulfur atoms attached to each boron and nitrogen atom along the cut edges. Under these conditions, the insulating boron nitride becomes metallic.
The team used two systems for the simulation. Jaguar ran the Vienna Ab-initio Simulation Package (VASP) and the Oak Ridge National Laboratory Institutional Cluster ran the SIESTA code. Both are density functional theory (DFT)-based codes.
“VASP needs the outstanding computational capabilities of Jaguar,” said Lopez-Bezanilla. “To verify the metallic features of the oxygenated zBNNRs with a higher degree of accuracy, we performed spin-polarized calculations with the help of VASP on large unit cells, which needed thousands of processors due to the extensive calculation of large systems at the level of a DFT code based on plane waves.”
Typical job sizes were 2,048 cores. “The project consisted of doing a large number of calculations at different levels of detail,” said team member Bobby G. Sumpter, group leader of Computational Chemical and Materials Science and the Nanomaterials Theory Institute at ORNL. “Jaguar was needed to perform certain calculations that used a different level of detail for the electronic structure—in particular to check system size effects and more rigorous treatment of electron exchange and spin, but also to examine the use of more complete basis sets. Basically Jaguar provides the required computational engine that enables us to do a complete study, without which we would not have been able to fully develop an understanding of the boron nitride nanoribbons within months.”
The computational models showed that the edge shape, the choice of added elements—oxygen, sulfur, or hydrogen—and the location of the elements all play a role in creating different behaviors in the nanoribbons.
The repetitive zigzag edge shape sets the added oxygen atoms at the same position—always parallel to the edge and equidistant from the adjacent oxygen atoms, so that the electron shells overlap, allowing electrons to move between atoms. In other words, it gives the nanoribbon metallic qualities. If BNNRs with armchair edges are modified, the oxygen atoms are arranged differently, preventing the emergence of metallic characteristics.
Both oxygen and sulfur render the zBNNRS metallic, whereas the control group of hydrogen-edged zBNNRs was semiconducting and nonmagnetic.
The placement of the elements also affects the metallic qualities and conductivity. “To show the importance of the edges [in converting] the zBNNRs into a metallic compound, we modeled the doping of the ribbon when the oxygen atoms form an extended wire embedded in the center of the ribbon,” says Lopez-Bezanilla. “The result is that it conserves its insulating features.”
The team outlined its findings in a July 7, 2011, paper in the American Chemical Society journal Nano Letters.
The simulation results have not yet been tested in a laboratory.
“So I encourage experimentalists: take zBNNR, put oxygen on the edges, and test what I said. We encourage chemists to do that, and if it is eventually synthesized, we will have some nanoelectronic applications,” says Lopez-Bezanilla.
Simulating the transformation of a known material into one with novel features opens the door to possible new uses – optical, magnetic, electronic. There is a new world of opportunities for the features in zigzag-edged boron nitride nanoribbons. Reproducing Lopez-Bezanilla’s results in the lab will put a smile on many faces.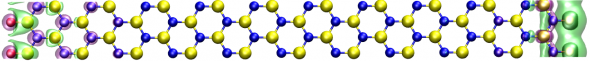
Related publications:
Alejandro Lopez-Bezanilla, Jingsong Huang, Humberto Terrones, and Bobby G. Sumpter. 2011. Nano Letters, 11(8), pp 32673273. doi: 10.1021/nl201616h
