A theoretical calculation has become an indispensable approach to revealing the thermodynamics and kinetics of catalysis. Static computational strategy is the most popular approach in theoretical catalysis, in which the reaction thermodynamics and kinetics are evaluated based on a few stationary geometries at zero temperature and some ideal statistic mechanics models. 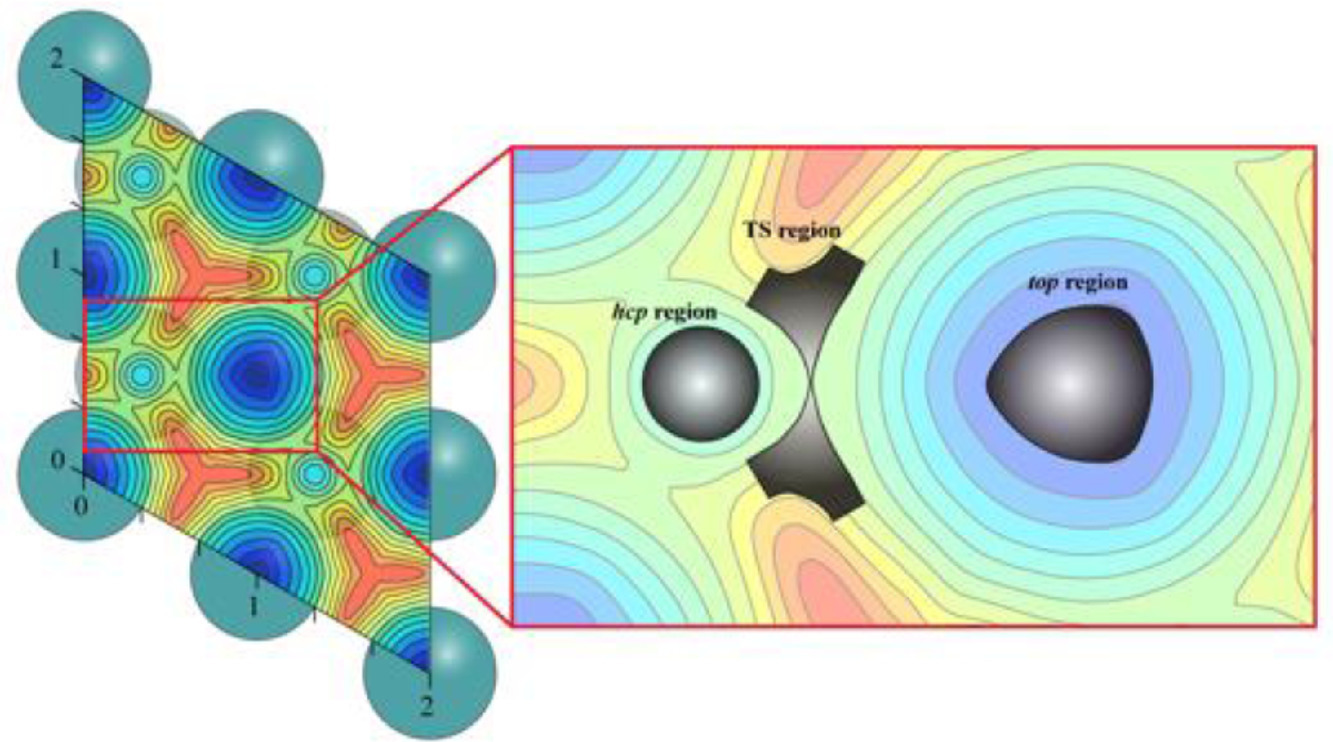
Therefore, the accuracy of the widely used static approach is limited by the compatibility between ideal models and realistic conditions. In comparison, ab initio molecular dynamics (AIMD) simulation is a well-tested approach going beyond static stationary states. However, it is very difficult to obtain converged thermodynamics and kinetics due to the significantly high computational cost for long-timescale simulations.
In a recent study published in Chinese Chemical Letters, Prof. CHEN Jun and Prof. CHEN Zhening from Prof. ZHUANG Wei's group at the Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences proposed a highly efficient dynamic computational strategy for the calculation of thermodynamics and kinetics in heterogeneous catalysis on the combination of efficient potential energy surface (PES) and molecular dynamics (MD) simulations.
Using chemisorbed CO on Ru(0001) surface as the illustrative model catalytic system, the researchers achieved a large time scale MD at the level of microsecond, and the neural network-fitted PES displayed a high efficiency.
They obtained reliable temperature dependent thermodynamics and kinetics at each of seven different temperatures ranging from 300 to 900 K, which goes beyond the popular static approach as well as the transition state theory, providing a more precise description of catalytic processes at realistic conditions.
Furthermore, the researchers indicated that the dynamic computational strategy based on efficient PES and MD simulations is readily available as an accurate yet efficient approach to evaluate the precise thermodynamics and kinetics of catalytic processes at realistic conditions. The results can be used as an important benchmark for subsequent study of dynamic protocols for heterogeneous catalysis.
This study clearly demonstrates the efficiency and reliability of the dynamic computational strategy based on efficient PES and MD simulations, which is expected as a powerful tool for the statistical thermodynamics and kinetics calculation in catalysis, in conjunction with the proper enhanced sampling approaches when necessary.


 How to resolve AdBlock issue?
How to resolve AdBlock issue?