GOVERNMENT
Jiménez-Osés lab's simulations show additional insights into the SARS-CoV-2 spike protein glycan shield
Never as these past two years manifested the importance of collaborative research in virology and immunology. Readiness of action when such striking pandemic events occur relies on decades of basic knowledge accumulated in time and constant technology development, which rely on stable scientific policies on a global scale. An unprecedented wealth of information has been gathered on SARS-CoV-2 in a very short period mainly focused on the cellular entry process and mechanism of antibody recognition where mainly protein-protein interactions occur. However, the SARS-CoV-2 spike protein is decorated by chains of carbohydrates (sugars) whose identity and flexibility have essential implications for antibody escaping, cellular proteins recognition and so for….
The groups of Dr. Abrescia and Dr. Jiménez-Osés at CIC bioGUNE in Spain, have combined high-resolution cryo-electron microscopy and supercomputer simulations to understand the correlation between sugar identity and flexibility in SARS-CoV-2 spike glycoprotein and published the results in Front Microbiol. on 15th of April 2022. 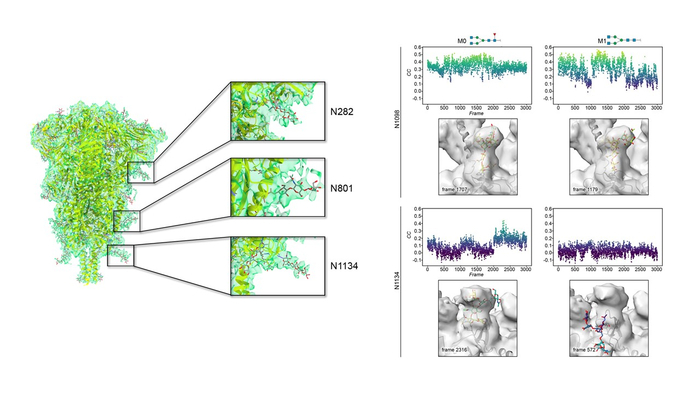
Rapid and free access to high-end Krios microscopes at eBIC-Diamond LS (UK) by the Abrescia Lab has allowed a 3D reconstruction of the spike protein at 4.1 Å resolution with a minimal number of contributing particles (~23,000) in which the density for the decorating glycans is as clear as other maps at a higher resolution for which hundreds of thousands of particles were necessary. The most ordered sugars are located in the so-called S2 domain which is proximal to the viral membrane (Fig. 1 left). Chemical variations of those glycans discovered by mass spectrometry were modeled on representative glycosylated amino acids by the Jiménez-Osés lab and showed no significant influence on either protein shielding or glycan flexibility. Mathematical methods were used to compare the cryo-EM density and the time-resolved full-atom supercomputer models. The best fits between the two techniques are characterized by glycans showing very conserved geometries around crucial glycosidic bonds near the protein (Fig. 1 right). Being able to predict glycan behavior is relevant because this S2 location on the spike is also the one mostly conserved across the other human coronavirus and the ideal target for a pan-ligand capable to neutralize the virus after cell entry. This study – also a result of collaborations with Jimenez-Barbero, Millet, and Connell labs - shows that experimental and computational tools combined can provide valuable insights into the conformational preferences of inherently flexible and complex glycoconjugates, advancing the discovery of new drugs able to evade the glycan shield of infectious viral proteins.
