INDUSTRY
Hershberg lab develops new data analysis, visualization tools to create atlas of specialized defense cells in the human body
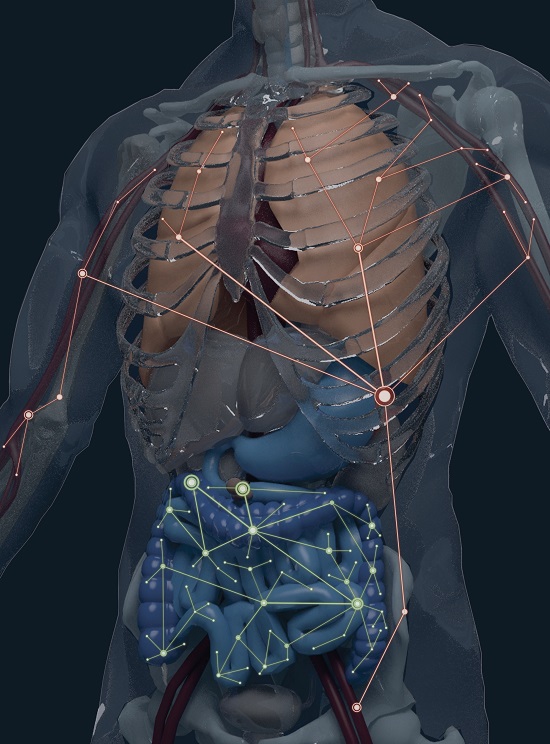
Drexel University computational biologists have helped to create the first “anatomic atlas” of B-cell clone lineages, their properties and tissue connections.
The researchers revealed that the population of B-cells — highly specialized immune cells that generate the antibodies that protect us from disease — are split into two broad networks within the body. One spans the blood, bone marrow, spleen and lung, while the other is found in the gastrointestinal tract. This atlas of B-cell tissue distribution, published in Nature Biotechnology, will be an essential resource for researchers and clinicians studying infectious diseases, cancer, the microbiome and vaccine responses.
“We have now shown that the geography of the human body is important for how the immune system works, with B-cells operating differently depending on where they are located,” Hershberg said.
To discover how B-cell clones are distributed in the body, the researchers sequenced 933,427 lineages and mapped them to eight different anatomic compartments in six human organ donors.
The main challenge the researchers faced was to prove that if they did not find B-cells in a certain region of the body, that they were not actually there — the same way an ecologist must prove the extinction of an animal species, according to study co-corresponding author Uri Hershberg, PhD, an associate professor in the School of Biomedical Engineering, Science and Health Systems at Drexel.
“B-cell clones are highly diverse. We have over 100 billion different B-cell types at any time. So even at 1 million B-cells sequenced, we definitely did not sample all of them,” Hershberg said. “That is why our group used statistical tools from ecological research to estimate how many clone types were missing from our survey. When considering large experienced B-cell clones, we had sampled enough to prove with high confidence that they are segregated to the blood or the gut.”
B-cells are key players in the body’s protective immunity. When your body is infected by a particular pathogen, only the specific B-cells that recognize the invader will respond. These specialized cells then quickly multiply and create an infantry of identical sister cells of a specific clono-type to fight the infection. Special types of B-cells “remember” the invader, making you immune to a second attack.
The tissue distribution and trafficking of these cells from the same clone are essential to understand, since these processes influence how infections are controlled throughout the body. Animal studies have indicated that the tissue localization of B- cells and plasma cells is important for protective immunity and the homeostasis of bacterial microflora. However, unlike lab mice, humans live for decades in diverse environments and are exposed to many different pathogens.
To understand how B-cell clones are distributed in the human body, the scientists needed both donated human organs, as well as new data analysis and visualization tools developed by the Hershberg lab at Drexel.
Co-author Donna Farber from Columbia University directed the organ donor tissue program responsible for acquiring the necessary tissue samples from six individuals. Researchers at the University of Pennsylvania’s Perelman School of Medicine, led by Eline T. Luning Prak, traced and sequenced the B-cell populations in eight tissues across these individuals. In one case, they collected and sequenced B-cells from 257 different samples, which resulted in over 23 million different sequences.
At Drexel, Hershberg and his team of biomedical engineers generated and organized the sequences into comprehensive data sets, creating figures that showed the diversity, similarity and networks of large clones. They then built a database system, which can now be used by other scientists to standardize how this type of data is analyzed in future studies.
The researchers’ findings may help identify tissue-specific markers for B-cells, Hershberg said.
“Our findings suggest that there are certain kinds of B-cells in the gut that are not found in the blood, which is important because diseases tend to happen at the interfaces of your body — the lungs, the gut the throat. However, those organs are more difficult to test than the blood,” he added. “If we can identify what is ‘special’ about the B-cell clones in the gut, then we can look for those same markers in the blood.”
PhD candidate Bochao Zhang, postdoctoral researcher Gregory W. Schwartz and software engineer Aaron M. Rosenfield also contributed to this research.
