INDUSTRY
Johns Hopkins researchers develop model estimating the odds of events that trigger sudden cardiac death
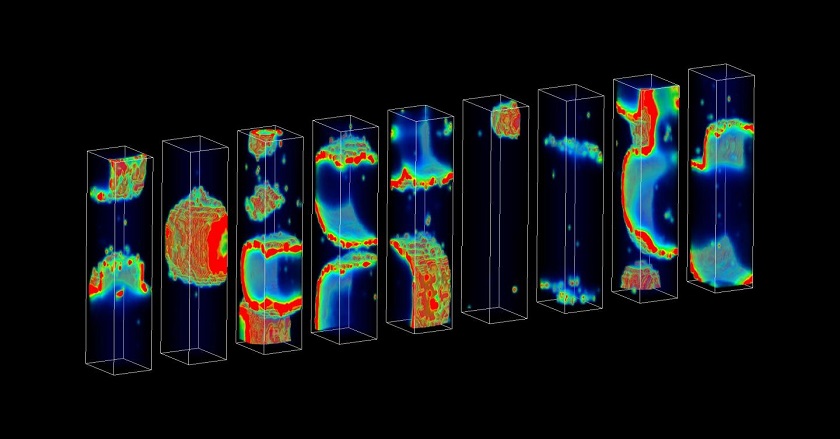
Supercomputational tool could uncover molecular underpinnings of rare, deadly arrhythmias
A new supercomputational model of heart tissue allows researchers to estimate the probability of rare heartbeat irregularities that can cause sudden cardiac death. The model, developed by Mark Walker from Johns Hopkins University, is presented in PLOS Computational Biology.
An increased risk of sudden cardiac death is associated with some heart diseases. It occurs when an irregular heartbeat (arrhythmia) interferes with normal electrical signaling in the heart, leading to cardiac arrest. Previous research has shown that simultaneous, spontaneous calcium release by clusters of adjacent heart cells can cause premature heartbeats that trigger these deadly arrhythmias.
Despite their importance, arrhythmias that can cause sudden cardiac death are so rare that estimating their probability is difficult, even for powerful high performance computers. For instance, using a "brute force" approach, more than 1 billion simulations would be required to accurately estimate the probability of an event that has a one in 1 million chance of occurring.
In the new study, the researchers developed a method that requires just hundreds of simulations in order to estimate the probability of a deadly arrhythmia. These simulations are powered by a supercomputational model that, unlike previously developed models, realistically incorporates details of the molecular processes that occur in heart cells.
The researchers demonstrated that, by altering model parameters, they could use the model to investigate how particular molecular processes might control the probability of deadly arrhythmias. They found that specific, molecular-level electrical disruptions associated with heart failure increased the probability of deadly arrhythmias by several orders of magnitude.
"This study represents an important step forward in understanding how to pinpoint the molecular processes that are the primary regulators of the probability of occurrence of rare arrhythmic events," says study co-author Raimond Winslow. "As such, our approach offers a powerful new computational tool for identifying the optimal drug targets for pharmacotherapy directed at preventing arrhythmias."
"Multiscale [super]computer models are critical to link an improved understanding of drug-disease mechanisms to tissue and organ behavior in complex diseases like heart failure," says Karim Azer, Sr. Director and Head of Systems Pharmacology, Sanofi, who was not involved in the study. He added, "The models provide predictions that can be tested in the laboratory or the clinic, and as such, the pharmaceutical industry is increasingly utilizing mathematical and computational modeling approaches for enabling key drug discovery and development decisions regarding drug and patient characteristics (i.e., towards precision medicine)."
In the future, the team plans to apply their method to three-dimensional cell clusters, instead of the one-dimensional fibers explored in this study. Future work could also employ experiments with engineered human cardiac tissue to help verify the model's predictions.
